US FDA approves Cognota's BP monitor device

US FDA approves Cognota's BP monitor device
Cognota can export its blood pressure monitor devices to the US, Europe, and other overseas countries
/smstreet/media/post_banners/bqRdggRa8kRjIDxgKkht.jpeg)
Cognota Receives US FDA Approval for BP Monitor Device
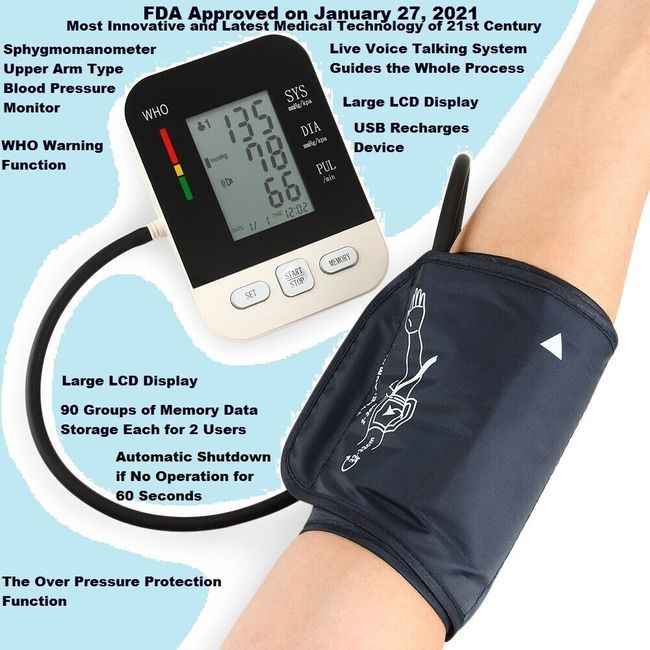
Systolic & Diastolic Blood Pressure Readings Memory Recall up to 90 readings Irregular Heartbeat detector Hypertension Detector Heart rate reading

Clinical Automatic Blood Pressure Monitor FDA Approved by Generation Guard with Portable Case Irregular Heartbeat BP and Adjustable Wrist Cuff Perfect

Cuff Free Blood Pressure Monitor Approved By FDA

FDA Approved Fully Automatic Upper Arm Blood Pressure Monitor 3 Mode 4 Cuffs Electronic Sphygmomanometer

FDA Approved Smart Upper Arm Blood Pressure Monitor

Sanjeev Dahiwadkar on LinkedIn: cognota-receives-us-fda-approval-for-bp- monitor-device.pdf

Cognota Healthcare - Company Profile - Tracxn

Story Behind the Rise of the World's First FDA-Approved Wearable Blood Pressure Monitor

Blood Pressure Archives - BioVoiceNews
Cognoa Receives FDA Breakthrough Designations for Autism

CardiAi Inc. launches BPAro a Brand New FDA & Health Canada approved Clinically validated 24-hour Ambulatory Blood Pressure Monitoring System with all new features including Bluetooth, Diabetes setting & Auto Interpretation of